Introduction to Quantum Physics
Quantum physics is a branch of physics that deals with the study of the behavior of matter and energy at a very small scale. It is a fundamental theory that explains the behavior of subatomic particles such as electrons, protons, and neutrons, which make up the building blocks of matter. Unlike classical physics, which deals with the laws of motion and the behavior of macroscopic objects, quantum physics deals with the laws of probability and the behavior of particles at the quantum level.
Historical Development of Quantum Physics
The development of quantum physics can be traced back to the late 19th century when scientists such as Max Planck and Albert Einstein were studying the behavior of light and electromagnetic radiation. In 1900, Planck proposed that the energy of electromagnetic radiation is quantized, meaning that it can only exist in discrete packets of energy known as quanta. This idea was revolutionary at the time and formed the basis for the development of quantum mechanics. In 1905, Einstein further developed the concept of quanta by proposing that light is made up of particles called photons, which have both wave-like and particle-like properties. This idea helped to explain several phenomena, such as the photoelectric effect, which was observed when photons of light knocked electrons off a metal surface. In the 1920s, a group of physicists including Niels Bohr, Werner Heisenberg, and Erwin Schrödinger developed the mathematical framework of quantum mechanics, which provided a way to predict the behavior of subatomic particles. This framework was based on the principle of wave-particle duality, which states that particles can exhibit both wave-like and particle-like behavior depending on how they are observed.
The Principles of Quantum Mechanics
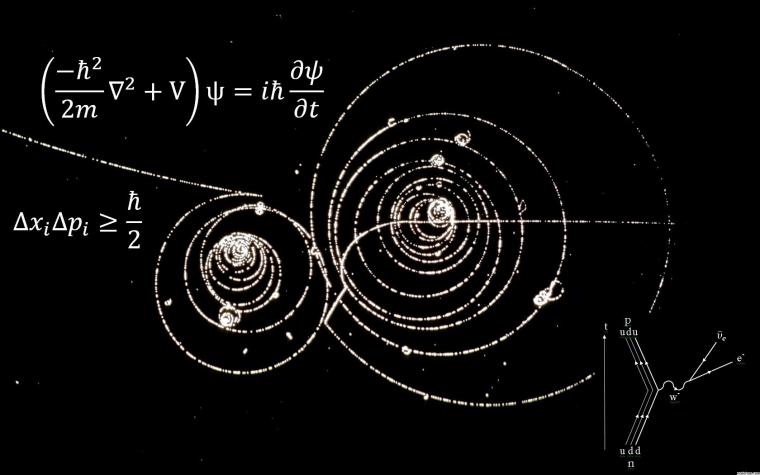
Quantum mechanics is based on several principles, including the principle of superposition, the uncertainty principle, and the principle of entanglement. The principle of superposition states that a particle can exist in multiple states at the same time until it is observed or measured. For example, an electron can exist in multiple energy levels around an atom until it is observed and collapses into a specific energy level. The uncertainty principle, proposed by Heisenberg, states that it is impossible to know the position and momentum of a particle simultaneously with absolute precision. This is because the act of measuring one property of a particle affects the other property, making it impossible to know both properties at the same time. The principle of entanglement, proposed by Schrödinger, states that particles can become entangled, meaning that their properties become correlated even if they are separated by a great distance. This idea has been demonstrated in several experiments, including the famous EPR (Einstein-Podolsky-Rosen) experiment, which showed that particles can influence each other's properties even when they are separated by a large distance
Applications of Quantum Mechanics
Quantum mechanics has several applications in modern technology, including the development of transistors, lasers, and cryptography. The principles of quantum mechanics have also led to the development of new fields of research such as quantum computing and quantum communication. Quantum computing is a field that explores the use of quantum mechanics to perform calculations that would be impossible with classical computers. This is because quantum computers can perform calculations using multiple states simultaneously, which allows them to solve problems that would take classical computers millions of years to solve. Quantum communication is a field that explores the use of quantum mechanics to transmit information securely over long distances. This is because the act of measuring a particle changes its properties, making it impossible for an eavesdropper to intercept the information without being detected.
Conclusion
Quantum mechanics is a fascinating field of study that has revolutionized our understanding of the behavior
Comments
Post a Comment